When we think of hybrid clinical trials Design, we usually picture traditional randomized controlled trials (RCTs) where participants are allocated to one of two treatment groups (standard or experimental) under the close watch of clinicians and healthcare professionals. Participants are monitored frequently for vital signs and adverse events in an in-clinic setting or central site to assess the efficacy and safety of the new treatment giving a view of a strict, highly restrictive environment, with limited accessibility and narrow patient criteria.
Limitations of traditional clinical trials include difficulties in patient recruitment and retention, high site maintenance costs, and the inability to project trial outcomes to a wider and more diverse patient population. These drawbacks of traditional trial designs have led to the introduction of alternative methods to conduct clinical trials.
Recently, the clinical trial landscape is filled with buzzwords such as virtual, decentralized, and hybrid clinical trials. Although these trial designs have existed for a reasonably long time, the impetus for the widespread adoption of these trial designs has been the COVID-19 global pandemic.
So, what are Virtual, Decentralized, and Hybrid clinical Trials, and why are they becoming so popular?
The Clinical Trials Transformative Initiative (CTTI) defines decentralized clinical trials (DCT) as studies executed through telemedicine and mobile/local healthcare providers (HCPs), using processes and technologies differing from the traditional clinical trial model (1). The primary objective of decentralization is to either use digital tools such as telemedicine or electronic communications/platforms and bring clinical trial processes such as consent and assessments closer to patients. In fully decentralized trials (also known as virtual or siteless trials all clinical trial activities from study start-up, patient recruitment and enrolment, patient monitoring, investigational product (IP) dispensing, and administration, data capture, and data analysis are carried out remotely.
There is no face-to-face contact between study participants and healthcare professionals with all activities carried out on an electronic platform. Virtual trials are rare and can only be done for drugs that have a well-defined safety profile making this trial design of limited applicability. Furthermore, the lack of human or social contact in virtual trials may not be preferable for all patients who associate hybrid clinical trials with interaction and increased care provided by health care professionals (HCP). Despite this, the advantages of decentralization are many fold-they allow for easier patient recruitment with a diverse population that mimics the real-world scenario, improved patient accessibility to novel treatments, reduced costs associated with site maintenance, real-time monitoring of patient data allowing for faster and more efficient care and increased patient engagement.
Considering the benefits of decentralization, hybrid trials are becoming increasingly popular. Hybrid clinical trials incorporate elements of both in-clinic and fully decentralized trials, using the traditional approach for some trial activities and a virtual approach for others (Figure 1). This type of trial design is ideal to improve data capture and retain patient engagement while using the on-site or in-clinic elements for some aspects of the study.
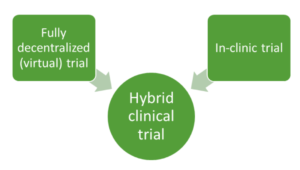
Figure 1. Hybrid trials involve a mix of elements of in-clinic and full decentralized (virtual) trials.
The main driver for the interest in hybrid clinical trials is the patient-centric approach to clinical trials with regards to patient ease and accessibility. Other factors that are making hybrid trials popular are increased comfort with the use of digital tools and technology such as heart rate monitoring devices by patients. Sponsors are becoming increasingly comfortable with the decentralization approach as regulatory bodies are accepting the use of digital health technology and digital endpoints for certain trials.
The COVID-19 pandemic has further emphasized the importance of decentralization as it is expected to overcome hurdles associated with patient recruitment and engagement caused by lockdown measures and travel restrictions allowing for continuity of the trials. In 2021, the Food and Drug Administration (FDA) released guidance on the application of digital health technology (DHT) in clinical trials to allow for remote data collection This guidance emphasized the importance of clearly understanding the appropriateness of a DHT in clinical investigations and its associated risks and management (2).
Strategy for using a Hybrid Clinical Trials Design
Like all clinical trials, the objective of a hybrid clinical trials is to demonstrate the safety and efficacy of a new treatment versus a standard comparator. Keeping this goal in mind it is necessary for the sponsor of a hybrid clinical trials to consider the following factors in developing a strategy around the deployment of virtual tools that would be useful at various stages of a trial. An effective strategy takes into consideration the patient-specific and operational characteristics of the trial in conjunction with regulatory requirements. The following points should be considered when developing a hybrid clinical trials (Figure 2):
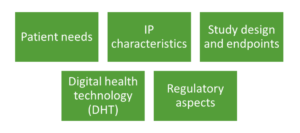
Figure 2. Various factors to consider in a hybrid clinical trials
Patient Needs:
Understanding the patient population is an important factor in deciding whether digital tools would be beneficial for use in a trial and what tools would be appropriate depending on the stage of the trial. Various factors related to the patient such as age, mobility, medical conditions, and familiarity with technology need to be considered. Elderly patients may benefit from the reduced burden of on-site visits by digital technology but may fear using these tools making it important to use easier DHT tools for them compared to younger, technology-savvy patients. Consenting is an important step in any trial and when using the digital consenting process, it is important to consider the patient population and the level of detail necessary so that patients are comfortable and remain engaged throughout the study. Social media and marketing may help to alleviate some difficulties in patient recruitment and retention. Patient engagement mobile application may be improved if patients can use technologies such as mobile phones and watches that they are familiar with and they have easy accessibility to all trial-related personal documents such as consent forms and test results. Further, introducing some level of human contact by local doctors or nurses that can visit patients at their homes for some tests and assessments can make patients comfortable with the trial process. Troubleshooting services and direct connection with healthcare professionals or investigators that can help answer any patient concerns are required for certain patient populations.
Investigational Product (IP) Characteristics:
The safety profile and level of knowledge available about the IP is also a deciding factor in choosing whether to allow for drug administration in local clinics or at home by a nurse or technician (decentralized approach) or whether drug dosing should be done at a trial site by trained professionals who can closely monitor the patient for any adverse reactions. Ease of administration and storage requirements of the IP should also be considered. In many cases, the first dosing is done at a site under the close supervision of medical staff followed by subsequent doses being given at local clinics or self-administered by patients.
Study Design and Endpoints:
The decision to use digital health technology (DHT) to collect patient data for trial endpoints depends on the complexity of the trial. Trials that do not require professional assessments are more amenable to the use of digital technology via wearables or electronic patient-reported outcomes (ePRO) or telemedicine approaches. Whereas those that require frequent assessments using specialized equipment may need to be performed in clinics or at trial sites.
Digital Health Technology (DHT):
The type of DHT (sensors, software, or combination of both) that is to be used in a trial and the rationale for its use should be clearly explained (fit-for-purpose) in a submission. Various factors like patient population, design, and operation of DHT are important to consider.
Regulatory Aspects:
It is necessary for the sponsor of hybrid or decentralized trials to be well-versed with local and regional regulations on the acceptability of DHT and virtual tools for obtaining patient consent and for trial assessments. Submissions should also include data on validation and verification studies for DHT which can be provided by the manufacturer.
Therefore, it is essential for sponsors to consider all the above factors in deciding to where best use a decentralization approach versus a traditional site-based approach for various steps in a clinical trial. Answering the questions of the value of decentralization at each step and DHTs needed for it along with patient involvement such as training on technologies are pivotal to the success of a hybrid trial.
Virtual tools in Hybrid Clinical Trials
The decentralization component of hybrid clinical trials requires the sponsor or contract research organization (CRO) to have an arsenal of digital tools and platforms for the seamless management of the trial. Specific factors such as an increase in the number of local sites or patient training programs, data security and dissemination issues, and patient and investigational product (IP) management differ considerably between in-clinic and decentralized trials and need to be considered.
The following are the common virtual tools required to perform various steps in a clinical trial using a decentralized approach:
Patient Engagement
- Patient consent: electronic consent (eConsent), electronic signatures (DocuSign)
- Patient recruitment: virtual prescreening, social media, and marketing platforms
- Patient education and support: patient portals, video-recorded training programs, telephonic helplines
- Patient adherence: SMS reminders, e-mail reminders
Site Management
- Staff training: video presentations, pre-recorded online courses
- Remote monitoring: electronic data capture (EDC) platforms, clinical trial management system (CTMS) tool for schedules, contracts and payments, documents, milestones, clinical trial site activities, workflows, and data analytics
- Risk management: virtual risk management tools using spreadsheets
- Documentation:electronic trial master file (eTMF)
Trial Supply
- Direct-to-patient or direct-to-site shipping
- Interactive response technology (IRT) to record investigational product (IP) and kit assignments
- GPS and tracking software
- Temperature and humidity logging software
Assessments and Monitoring
- Telemedicine platforms and smartphone/tablet applications
- Electronic clinical outcome assessment (eCOA) and electronic patient-reported outcomes (ePRO)
- Electronic case report forms (eCRF)
Hybrid clinical trials allow melding the best of both in-clinic and decentralized or virtual trial models. The rise of hybrid clinical trials is in tune with technology and digital platforms such as wearable technology, sensors, and cloud computing. Leveraging the benefits of hybrid clinical trials can save sponsors money, increase patient engagement, and help collect data that is more representative of real-world settings.
References:
- CTTI Recommendations: Decentralized Clinical Trials September 2018
- Digital Health Technologies for Remote Data Acquisition in Clinical Investigations | FDA

