The steady and progressive shift of the clinical trial landscape to incorporate the Patient Centric Clinical Trials is a culmination of several factors such as digitization, artificial intelligence, increased patient awareness, access to mobile devices, and the more recent COVID-19 global pandemic. Unlike the traditional clinical trial paradigm that is centered around sites and sponsors, patient-centric trials are targeted towards patient comfort and convenience all the way from trial inception to data collection, and close-out. The site-centric approach to clinical trials is limited for several reasons:
- Insufficient patient recruitment due to the ability to recruit only those that live close to trial sites thus limiting generalizability and diversity
- High dropout rates and inability to retain patients caused by inconvenience to the patient’s daily activities by site assessment visits and complex dosing regimens
- Lack of personal communication and explanation of trial protocols to patients causing hesitation to participate in trials
- Inability to retain proper patient records at the time of occurrence of adverse events leading to errors or eliminations in record-keeping
- Patient assessments at predetermined time points that leads to incomplete knowledge of patients’ health status between site visits
To overcome limitations inherent in the site approach to clinical trials, virtual or decentralized clinical trials are becoming popular. These trials involve a ‘site-less’ approach to trial activities wherein some or all the activities such as recruitment, consent, dosing, assessments, and data collection are conducted remotely. This model has proved especially useful during the pandemic when travel restrictions and social distancing measures were in place thereby banning patients from visiting central trial sites. In addition to allowing trials to go on seamlessly during the pandemic, the decentralized approach to clinical trials opened avenues that allowed patient comfort to be considered leading to the concept of ‘patient-centricity’. Now despite several sites opening up and the ease of travel and better control of the virus, many sponsors are continuing to use the decentralized approach as it helps save costs related to site maintenance while allowing for better data collection and improved patient centric clinical trials adherence and retention. Patient centric clinical trials also include those that involve precision or personalized medicine which involves the development of drug treatments that are specific for certain groups depending on genetic make-up, lifestyle, and medical history. Despite the wide range of definitions for the term, patient centric clinical trials in all cases is targeted toward the patient and caregiver’s convenience and comfort.
Various drivers for the interest in patient centric trials include:
- Access to mobile devices and wearable technology such as wearable sensors and devices as well as increased patient awareness and comfort with them that can allow for the collection of large amounts of data without interfering with patients’ daily life
- Ability to process the captured data using artificial intelligence and data mining tools and make meaningful decisions regarding patient care
- Increased patient interest and knowledge that makes them part of the decision-making process and improves transparency in their treatment
- Improved ability to project clinical trial data to the real-world setting
The value and utility of patient centric clinical trials can only be realized when suitable tools are available to help fulfil all the requirements for clinical trials. The figure below shows the various ways digital technology can be harnessed to serve patients and thereby improve the conduct of patient centric clinical trials.
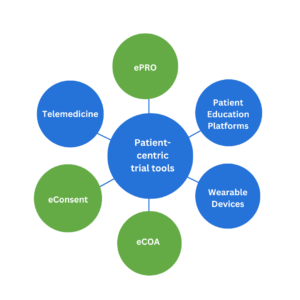
Figure. Various digital tools used in patient-centric clinical trials
-
Electronic patient-reported outcomes (ePRO) used in patient centric clinical trials:
Electronic Patient-reported outcome reports are an essential element of incorporating the patient’s voice into patient centric clinical trials as it involves a patient’s direct interpretation of their health status without any interference by physicians or study staff. The United States Food and Drug Administration (FDA) has released a guidance document that describes PRO instruments that can be used to support label claims (1). PRO instruments can be questionnaires that capture information from patients on symptom severity, quality of life, pain scores, etc. Traditional PRO instruments are paper-based and require the patient to answer the questions by filling in forms. Although these diaries or forms do contain information directly from the patient, they often do not capture complete data. Patients often fail to report symptoms at the time of occurrence due to the cumbersome nature of form filling. Electronic PROs (ePROs) are a better alternative to paper-based forms and may be designed as apps or e-patient diaries. They allow for real-time entry of the patient’s health status and remote real-time monitoring by physicians. They can be used to track patient’s symptoms, dosing, side effects, and other information on smartphones, tablets, or other electronic devices which are familiar to them and allow for data entry in the comfort of their own home. Often ePROs are equipped with reminders that alert the patient to enter information ensuring data capture in a timely manner. ePROs are expected to increase patient engagement and compliance as they enable patients to be involved in their own health.
-
Electronic consent (eConsent)used in patient centric clinical trials:
Informed consent from patients to participate in clinical trials is of utmost importance for the ethical conduct of patient centric clinical trials. To consent to participation, potential patients are required to fill in forms that often contain technical jargon and complex wording that patients are often not familiar with deterring them from participation and causing recruitment issues. Also, it is expected that the patients complete the required forms without leaving them any time to ask questions. In today’s day of increased patient education and awareness, it is necessary for patients to understand the patient centric clinical trial process and what is required of them. Electronic consent (eConsent) involves the use of electronic platforms such as interactive multimedia components that help patients to make informed decisions by presenting trial information in a simplified and engaging style that can be understood by patients and completed at their own pace. Various tools such as images, audios, videos, diagrams, and quizzes are included in eConsent forms that make the process more engaging for patients whilst ensuring that they understand their role in the patient centric clinical trial. In addition to providing a plethora of information in an understandable format to patients, eConsent also allows to maintain audit trails and ensure regulatory compliance (2).
-
Electronic clinical outcomes assessment (eCOA) used in patient centric clinical trials:
Collection of patient data such as pain scores or severity, and frequency of occurrence are used in conjunction with laboratory test results to understand the effect of treatments during a trial and make decisions. In contrast to traditional clinical outcomes assessment forms that involve transcription of information on paper, eCOA clinical trials involves the use of smartphones, tablets, and computers to record outcomes by patients, clinicians, and caregivers. eCOAs can include information that comes directly from the patient (ePRO), reports from the healthcare professional (clinical reported outcomes or ClinRO), and observations by caregivers (observer reported outcomes or ObsRO). Like ePROs, eCOAs and Patient Centric Clinical Trials allow for flexibility in data entry remotely and improve patient retention.
Incorporating some elements of patient centric clinical trials is becoming commonplace due to digital and mobile technologies thus improving the conduct of trials from both patients’ and sponsors’ perspectives. Although several tools are already in place to facilitate patient involvement and adherence, a paradigm shift in the adoption of electronic platforms still requires a change from the traditional mindset of paper-based approaches and training of sponsors in the correct implementation of these systems.
References

